Cellular Senescence and the Role of GLF16
GLF16 is a fluorophore-conjugated reagent enabling rapid detection, isolation and live tracking of senescent cells.
Cellular senescence, a stress response mechanism, is beneficial on a transient basis while its persistence has been related to aging and various pathologies, including cancer and neurodegenerative diseases (Cell 2019, Physiol Rev. 2023, J Pathol 2023, Cell 2024).
Since 1995, SA-β-Gal staining was the only available method to detect senescent cells (PNAS 1995). Yet, it has a limited range of substrate applications, is non-applicable in fixed material and prone to false positive and negative results (Exp Gerontol 2005, Aging Cell 2006).
Recently, exploiting lipofuscin as a hallmark of senescence, a compound under the trade mark SenTraGorTM was developed that reacts with this component, allowing accurate senescence detection in any kind of biological sample, including archival material (Aging Cell 2017, Cell 2019, Nat Protoc 2021). As SenTraGorTM needs to be diluted in ethanol, certain applications, like isolation of senescent cells via flow cytometry followed by their down-stream analysis (particularly omics application), have limited efficiency. Also, identification of live senescent cells is not feasible with this compound.
This limitation has been bypassed by the generation of GLF16, a water-soluble and lipofuscin-reacting compound (Mol Cell 2013). GLF16 is a robust fluorescent compound compatible with a wide range of methods for in vitro detection and isolation of senescent cells from various biological materials. Moreover, if packed in micellar nano-carriers it facilitates tracking and isolation of senescent cells in live organisms (Mol Cell 2013, STAR Protocols 2024). Recently, GLF16 has been included in the guidelines for senescence detection (Cell 2024).
References
- Cristofalo VJ. SA beta Gal staining: biomarker or delusion. Exp Gerontol. 2005 Oct;40(10):836-8.
- Dimri et al, A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995 Sep 26;92(20):9363-7.
- Evangelou et al, Escape from senescence: molecular basis and therapeutic ramifications. J Pathol. 2023 Aug;260(5):649-665.
- Evangelou et al, Cellular senescence and cardiovascular diseases: moving to the “heart” of the problem. Physiol Rev. 2023 Jan 1;103(1):609-647.
- Evangelou et al, Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 2017 Feb;16(1):192-197.
- Gorgoulis et al, Cellular Senescence: Defining a Path Forward. Cell 2019 Oct 31;179(4):813-827.
- Kohli et al, Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat Protoc 2021 May;16(5):2471-2498.
- Lee et al, Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006 Apr;5(2):187-95.
- Magkouta et al, A fluorophore-conjugated reagent enabling rapid detection, isolation and live tracking of senescent cells. Mol Cell. 2023 Oct 5;83(19):3558-3573.e7.
- Magkouta et al, One-step rapid tracking and isolation of senescent cells in cellular systems, tissues, or animal models via GLF16. STAR Protoc. 2024 Mar 15;5(1):102929.
- Ogrodnik et al, Guidelines for minimal information on cellular senescence experimentation in vivo. Cell 2024 Aug 8;187(16):4150-4170.
Description
GLF16 is a fluorophore-conjugated Sudan Black B (SBB) analogue tracing lipofuscin, a complex of proteins, lipids and metals formed as a consequence of translational deregulation during stressful insults and could be therefore considered the ‘’dark matter’’ of senescent cells1,2. Cellular senescence is associated with cell cycle arrest and deregulated metabolism, and therefore, is inextricably linked to macromolecular damage that leads to lipofuscin accumulation. Therefore, lipofuscin is one of the hallmarks of cellular senescence present in all types of senescent cells. Lipofuscin detection by GLF16 is a reliable, quick and straightforward method of senescent cells identification. Also, a micelle-based approach, mGLF16, allows for accurate detection, isolation, subsequent culturing and tracking of live senescent cells as well as the in vivo monitoring of senescence1,2.
Chemical Properties
| Chemical Name | 1-Ethyl-2-((E)-2-((E)-3-((E)-2-(1-ethyl-3,3-dimethylindolin-2-ylidene)ethylidene)-2-((4-(4-((2-methyl-6-((E)-(4-((E)-phenyldiazenyl)naphthalen-1-yl)diazenyl)-2,3-dihydro-1H-perimidin-2-yl)methoxy)-4-oxobutanamido)butyl)amino)cyclohex-1-en-1-yl)vinyl)-3,3-dimethyl-3H-indol-1-ium iodide |
|---|---|
| Molecular Weight | 1245.34 |
| Formula | C71H77IN10O3 |
| Excitation/Emission | 629 nm/743 nm
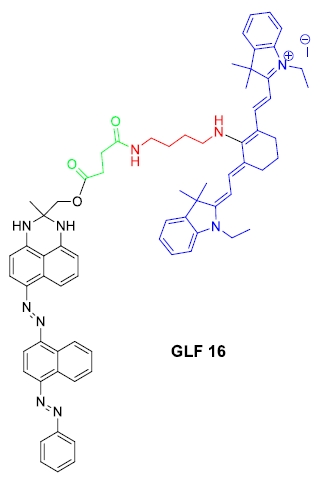
Figure 1. Chemical structure of GLF161. |
| Form | Blue-black colored solid, m.p. 173 – 5°C |
Applications
GLF16 can be applied on fixed cells as well as on tissue sections from frozen (OCT- embedded) or Formalin-Fixed and Paraffin Embedded (FFPE) samples. It can be used for Immunofluorescence (IF) and Flow Cytometry/FACS analysis.
The micelle enclosed GLF16, mGLF16, is recommended for Immunofluorescence (IF), Flow Cytometry/FACS analysis and live imaging of cells and animals.
In case of Ιmmunofluorescence (IF) staining use a GLF16 working solution of 50–70 μg/mL, while for Flow Cytometry/sorting analyses concentration may vary from 10 μg/mL (in case of large cells e.g., fibroblasts, tumor cells) to 2 μg/mL (for small cells with limited cytoplasm e.g., lymphocytes)1,2.
For Ιmmunofluorescence (IF) analyses of live senescent cells use 0.125 μg/mL of mGLF16 for 3 h (37°C, 5% CO2), while for Flow Cytometry/sorting incubate cells with 0.0166–0.166 μg/mL of mGLF16 for 3 h (37°C, 5% CO2). For the in vivo monitoring of senescence administer 1 mg of mGLF16 micellar dispersion (200 μL of the 5 mg/mL mGLF16 solution)1,2.
Published Data
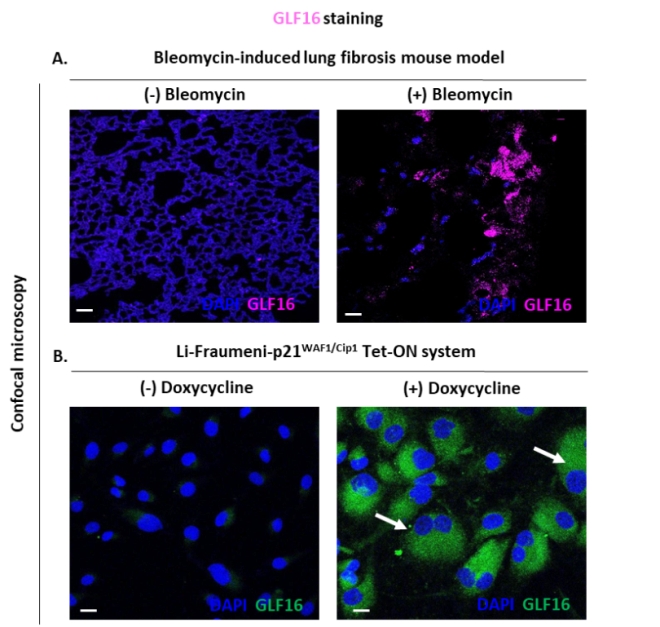
GLF16 staining of lung tissue sections and cellular models of induced senescence2.
(A) Representative pictures of GLF16 staining of lungs of bleomycin (right) or vehicle (left) treated mice. Objectives 103 and 403, Scale bars: 50 mm (-Bleomycin), 10 mm (+Bleomycin).
(B) Representative pictures of Li-Fraumenip21WAF1/Cip1 Tet-ON cells treated with doxycycline (right) or not (left). Lipofuscin aggregates are clearly visualized by GLF16, as depicted by white arrows. Objectives 403, Scale bar: 10 mm.
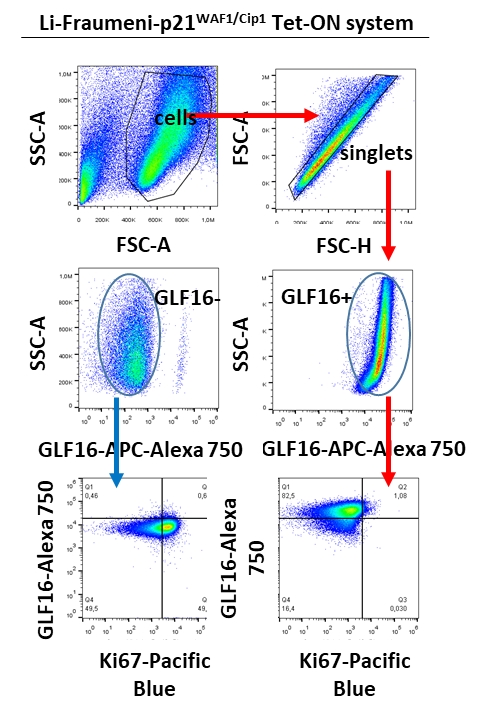
Figure 3. Flow cytometry application of GLF162.
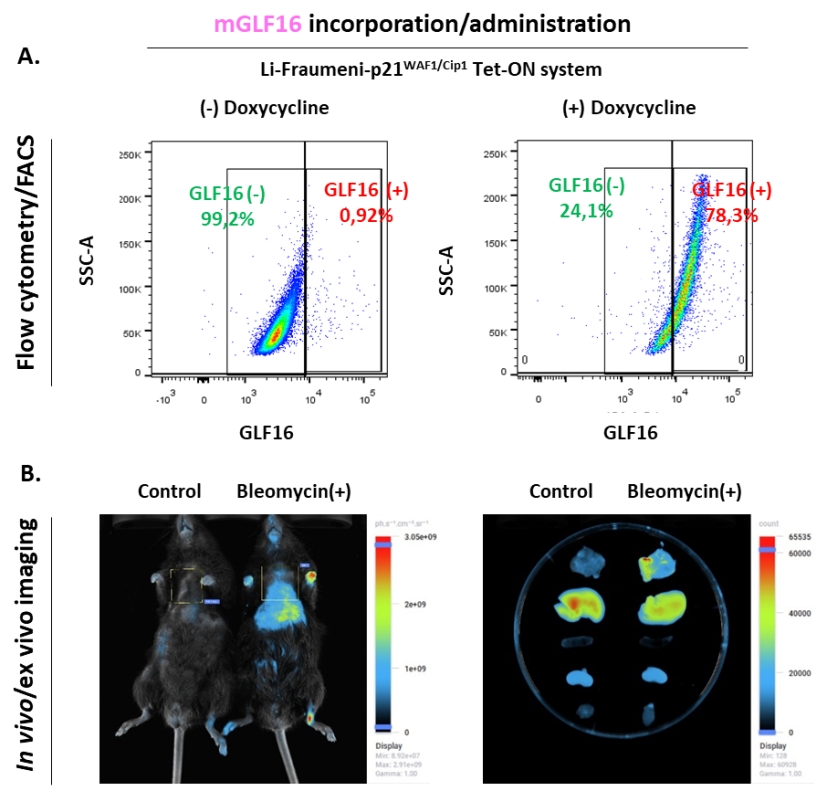
Figure 4. Representative results of mGLF16 application2.
(A) Representative flow cytometry analysis of cells incubated with m-GLF16.
(B) In vivo (left) and ex vivo (right) monitoring of senescence in the bleomycin-induced lung fibrosis model, Lu: Lung, Li: Liver, S: Spleen, H: Heart, K: Kidney.